✔ 100% Authentic Product

100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
Cashback offer(Optional):
- 🏷 Coupon CASH55 ✔️ Get ৳55 back - for Ordering ৳500+
- 🏷 Coupon CASH120 ✔️ Get ৳120 back - for Ordering ৳1200+
- 🏷 Coupon CASH200 ✔️ Get ৳200 back - for Ordering ৳2500+
- 🏷 Coupon CASH300 ✔️ Get ৳300 back - for Ordering ৳3500+
- 🏷 Coupon CASH400 ✔️ Get ৳400 back - for Ordering ৳4500+
- 🏷 Coupon CASH500 ✔️ Get ৳500 back - for Ordering ৳5500+
- 🔹 Delivery charge is applicable for Cashback offer
- 🔹 Foreign manufacturer products are not applicable for Cashback offer
Show More
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Urgent Delivery - 2 Hours Dhaka City
Urgent Delivery - 2 Hours Dhaka City ফ্রি ডেলিভারি! - ১৪৯৯ টাকা+ অর্ডারে ঢাকা
শহরে ।
ফ্রি ডেলিভারি! - ১৪৯৯ টাকা+ অর্ডারে ঢাকা
শহরে । ফ্রি ডেলিভারি! - ২৯৯৯ টাকা+ অর্ডারে ঢাকার
বাহিরে ।
ফ্রি ডেলিভারি! - ২৯৯৯ টাকা+ অর্ডারে ঢাকার
বাহিরে ।
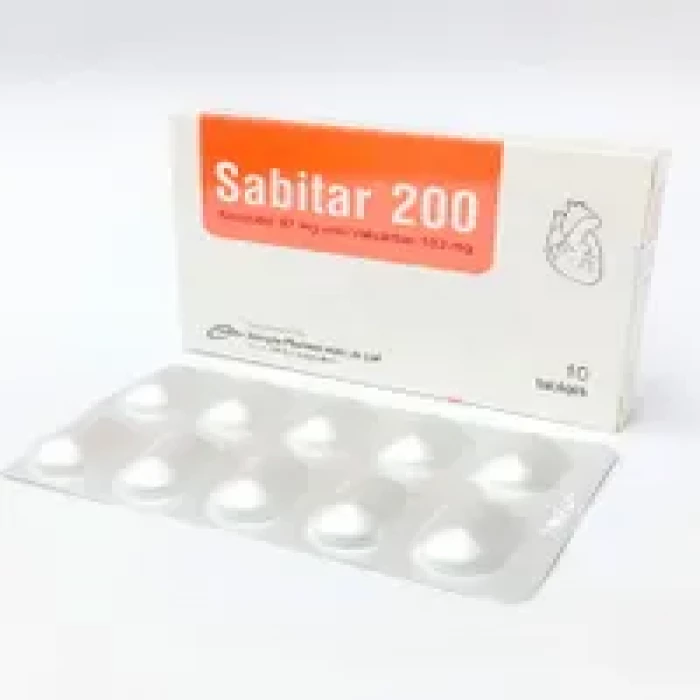
✅ Description:
Indications
In patients with chronic heart failure (NYHA Class II-IV) with a decreased ejection fraction, this combination is suggested to minimize the risk of cardiovascular mortality and hospitalization for heart failure.
In lieu of an ACE inhibitor or other ARB, this combination is generally given in conjunction with other heart failure treatments.
Pharmacology
The active metabolite of sacubitril, LBQ657, inhibits enkephalinase, a neutral endopeptidase that normally cleaves natriuretic peptides, including: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and c-type natriuretic peptide (CNP). ANP and BNP are released under atrial and ventricular pressure, activating subsequent receptors, leading to vasodilation, natriuresis, and diuresis. Under normal conditions, enkephalinase degrades other vasodilators and vasoconstrictor peptides, such as angiotensin I and II, endothelin 1 and amyloid beta protein. Therefore, neutral lysozyme inhibition leads to reduced degradation and increased concentration of endogenous natriuretic peptides, in addition to increased levels of vasoconstrictor hormones such as angiotensin II. (However, when used in combination with valsartan, it can cause angiotensin II to block its receptors, prevent vasoconstriction and cause a decrease in vascular resistance and blood pressure.) The cardiovascular and renal effects of sacubitril are elevated peptide levels As a result, these peptides are usually degraded by enkephalinase.
Valsartan is an oral medication that belongs to a class of drugs called angiotensin receptor blockers (ARB). It is a specific orally active angiotensin II antagonist that acts on the AT1 subtype. The binding of angiotensin to its receptors causes blood vessels to narrow (vasoconstriction), which leads to increased blood pressure (hypertension). Valsartan blocks angiotensin II receptors. By blocking the effects of angiotensin, valsartan can dilate blood vessels and lower blood pressure without affecting pulse rate. The affinity of valsartan for the AT1 receptor is much higher than that of the AT2 receptor (about 20,000 times). It does not bind to or block other hormone receptors or ion channels that are known to be important for cardiovascular regulation.
Dosage & Administration
The recommended starting dose of this combination is 49/51 mg twice-daily.
Double the dose of this combination after 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
Dose Adjustment For Patients Not Taking An ACE inhibitor Or ARB Or Previously Taking Low Doses Of These Agents.
A starting dose of 24/26 mg twice-daily is recommended for patients not currently taking an ACE inhibitor or an angiotensin II receptor blocker (ARB) and for patients previously taking low doses of these agents. Double the dose of this combination every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
Sacubitril & Valsartan is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to Sacubitril & Valsartan allow a washout period of 36 hours between administration of the two drugs.
Contraindications
This combination is not recommended:
Hypersensitivity to any component in patients
Having concurrent use of ACE inhibitors in individuals with a history of angioedema linked to previous ACE inhibitor or ARB treatment. Do not take within 36 hours of stopping or starting an ACE inhibitor.
In individuals with diabetes who are also on aliskiren,
Side Effects
Other parts of the labeling list clinically important adverse events, such as: Hyperkalemia, Angioedema, Hypotension, Impaired Renal Function
Pregnancy & Lactation
The active metabolite of sacubitril, LBQ657, inhibits enkephalinase, a neutral endopeptidase that normally cleaves natriuretic peptides, including: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and c-type natriuretic peptide (CNP). ANP and BNP are released under atrial and ventricular pressure, activating subsequent receptors, leading to vasodilation, natriuresis, and diuresis. Under normal conditions, enkephalinase degrades other vasodilators and vasoconstrictor peptides, such as angiotensin I and II, endothelin 1 and amyloid beta protein. Therefore, neutral lysozyme inhibition leads to reduced degradation and increased concentration of endogenous natriuretic peptides, in addition to increased levels of vasoconstrictor hormones such as angiotensin II. (However, when used in combination with valsartan, it can cause angiotensin II to block its receptors, prevent vasoconstriction and cause a decrease in vascular resistance and blood pressure.) The cardiovascular and renal effects of sacubitril are elevated peptide levels As a result, these peptides are usually degraded by enkephalinase.
Valsartan is an oral medication that belongs to a class of drugs called angiotensin receptor blockers (ARB). It is a specific orally active angiotensin II antagonist that acts on the AT1 subtype. The binding of angiotensin to its receptors causes blood vessels to narrow (vasoconstriction), which leads to increased blood pressure (hypertension). Valsartan blocks angiotensin II receptors. By blocking the effects of angiotensin, valsartan can dilate blood vessels and lower blood pressure without affecting pulse rate. The affinity of valsartan for the AT1 receptor is much higher than that of the AT2 receptor (about 20,000 times). It does not bind to or block other hormone receptors or ion channels that are known to be important for cardiovascular regulation.
Precautions & Warnings
Signs and symptoms of angioedema and hypotension should be observed
• Renal function and potassium level should be monitored in susceptible patients
Use in specific populations:
Pregnancy: Sacubitril/Valsartan can cause fetal harm when administered to a pregnant woman
Lactation: Drug should be discontinued during lactation
Pediatric use: Safety and effectiveness in pediatric patients have not been established
Geriatric use: No relevant pharmacokinetic differences have been observed in elderly (≥65 years) or very elderly (≥75 years) patients compared to the overall population
Hepatic impairment: Use not recommended
Storage Conditions
Store at room temperature between 20°C to 25°C. Protect from moisture.
Disclaimer:
ePharma sole intention is to ensure that its consumers get proper
information as musch as possible. Although we do not guarantee the
accuracy and the completeness of the information that provided and
here information is for informational purposes only.
The information contained herein should NOT be used as a substitute
for the advice of a qualified physician. This may not cover
everything about particular health conditions,
lab tests, medicines, all possible side effects, drug interactions,
warnings, alerts, etc. Please consult your healthcare professional
and discuss all your queries related to any disease or medicine. We
intend to support, not replace, the doctor-patient relationship.